This article grew out of a series of conversations among researchers at The Nature Institute during the current COVID-19 pandemic.1 In a matter of just a few months, an acute illness identified in a Chinese hospital in Hubei province has made its way around the world and brought viruses to the forefront of public consciousness. Beyond the many discussions we have had about this serious pandemic and the societal responses to it, we felt the need to give more attention to viruses as such. These “invisible germs” present a real riddle. What is an appropriate way to think about them?
We have been struck by the narrow way viruses are typically described—as enemies that attack us. Not alone among government leaders, President Emmanuel Macron of France declared his country to be “at war” with the virus. Is this an adequate way of viewing viruses and infectious diseases? We had a sense that it is not. The tendency to construct “us-them” dichotomies is all too prevalent in our times. It limits the breadth of dialogue, making it difficult to achieve deeper understanding. In the past few decades the traditional notion of bacteria as “bad germs” has been expanded by all the knowledge gained about the positive role bacteria can play, for example, in our microbiome.
So our central questions have been: To what extent has the way most of us have learned to picture viruses limited our ability to understand them and their place in the greater whole we call the earth? Can we gain a broader and more encompassing view? Here we share some of what we have found.
The Discovery of Viruses as Unseen Disease-Causing Agents
Long before the specific “agents” of infectious diseases were known, people knew that particular diseases were spread somehow through the environment and also from individual to individual. During the plague epidemic that decimated the population of Europe in the fourteenth century, sick individuals were quarantined.
But it wasn’t until the latter part of the nineteenth century that the germ theory of disease was established. Scientists discovered that certain bacteria could be isolated only from diseased individuals. These bacteria could then be cultivated in pure laboratory cultures. When they were injected into healthy animals that are susceptible to the disease, the animals would fall ill. Finally, the same species of bacteria could again be isolated out of these diseased organisms. If all these criteria were fulfilled, the bacteria could be considered the cause of the disease. While by no means uncontroversial at the time, the germ theory inspired many scientists to research known infectious diseases—in plants, animals, and humans—to see if they could find the bacterial cause. It was within this context that viruses were discovered.
In the 1800s, a disease of tobacco plants arose in which the leaves took on a mottled appearance and then became disfigured. In 1879 agricultural researcher Adolf Mayer began investigating the disease, which today is known as tobacco mosaic disease.2 He found that “the juice from diseased plants obtained by grinding was a certain infectious substance for healthy plants.” Evidently it was an infectious disease. Since Mayer was familiar with the germ theory, he inoculated tobacco plants with a variety of different bacteria, but the disease never appeared. He could find no evidence that bacterial or other tiny organisms were the infectious agents. After being heated to 80 degrees C (176 degrees F), the infectious fluid had no effect on the tobacco plants.
Independently, a Russian scientist, Dimitri Ivanovsky reported in 1892 that “the sap of leaves infected with tobacco mosaic disease retains its infectious properties even after filtration through [a] Chamberland filter.”3 The latter is an unglazed porcelain filter that was known to hold back bacteria. Since the fluid was still effective after passing through the filter, how could bacteria be involved? The observation was a riddle, but since both Mayer and Ivanovsky were strongly influenced by the bacterial germ theory, neither was ready to fully discount bacteria.
Carrying out further research, Dutch scientist Martinus Beijerinck found that a tiny amount of the fluid introduced through a syringe into a plant sufficed for the plant to become diseased. “If these diseased parts are extracted, an infinite number of healthy plants may be inoculated and infected from this sap, from which we draw the conclusion that the contagium, although fluid, reproduces itself in the living plant.” He spoke of a “contagium vivum fluidum”—a contagious living fluid that infected tobacco plants. The fluid therefore had the quality of something living, since it was proliferating and spreading from infected plant to infected plant. It was not some kind of toxin—which could of course damage or kill an organism—since a toxin does not increase in quantity within an organism. Beijerinck discovered that only those parts of the plant that were growing and undergoing cell division could be infected. He also observed that the fluid could not be cultivated on a nutrient medium in the laboratory, which can be done with most bacteria. The fluid could only increase within the living, growing tissue.
What Beijerinck discovered was a contagious living fluid with unique properties. He spoke, in his paper, of a “contagium” and also of a “virus.” The word virus comes from Latin meaning poison or poisonous fluid and was used in a variety of contexts in European languages at the time (his paper was in German).
This is an early discovery of a virus. At this time and for decades to come, the virus was not some thing one could see.5 It was an agency of a particular kind associated with fluids that had been passed through a bacterial filter that could infect and proliferate within a living organism. Also in 1898, German researchers Friedrich Loeffler and Paul Frosch found that foot-and-mouth disease in cattle and pigs could be induced by a fluid extracted from sick animals that had been passed through a bacteria filter and that, in contrast to bacteria, could not be grown on a nutrient medium outside the body.6 More and more viral diseases were discovered in the course of the coming decades. Virus-related human diseases include the common cold, flu, polio, small pox, rabies, chickenpox, HIV/AIDS, mumps, measles, and rubella.
The Virus as Particle
As scientists were discovering virus-related diseases in humans, animals, plants and bacteria, there was the widespread conviction that a virus must be some kind of tiny entity. How could a “mere” fluid reproduce? In 1935 the American chemist Wendell Stanley succeeded—through an involved chemical process—in forming crystals out of the juice of plants with tobacco mosaic disease. Highly diluted solutions made from the crystals maintained the ability to induce tobacco mosaic disease.7 He believed the crystals were protein and soon thereafter it became clear that the viruses consisted of both protein and nucleic acids (DNA or RNA).8
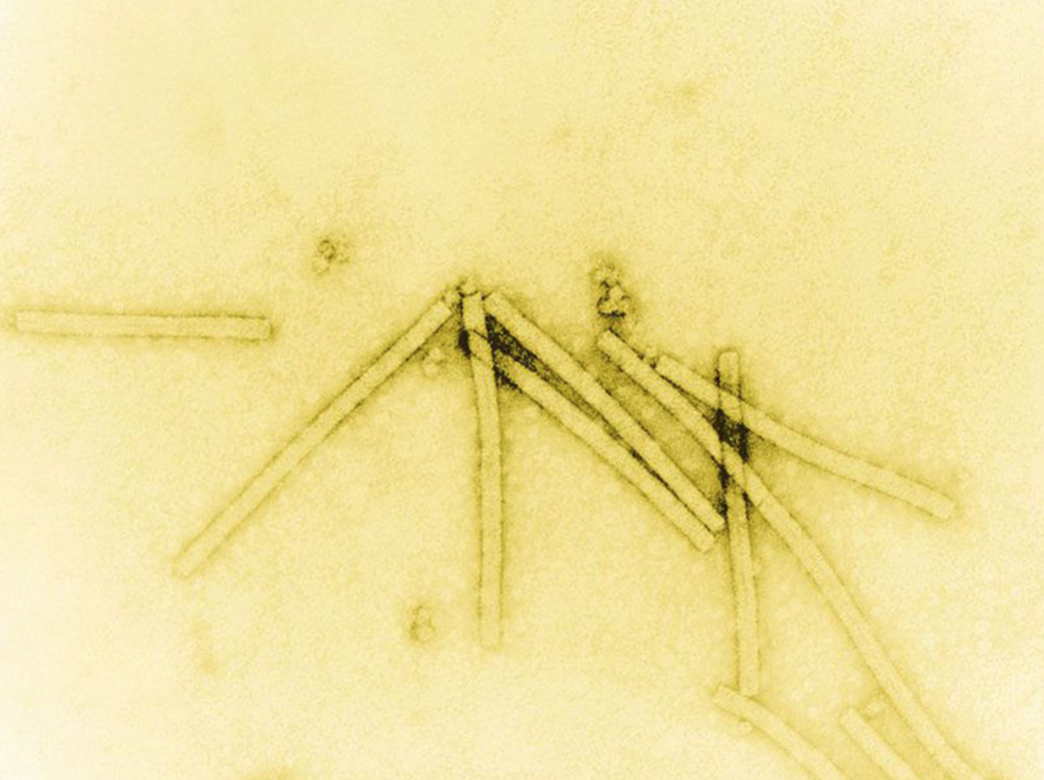
Researchers had to wait for the invention of the electron microscope, which allowed much greater magnification than light microscopes, to “see”—which means in this case to create an image of—virus particles. The first electron microscopic image of virus particles were made with the tobacco mosaic virus and achieved by letting gold adhere to what becomes visible as tiny particles.9 The tobacco mosaic virus particle shows the structure of a rod (see in a recent electron microscopic image below).
It is impossible to imagine in any concrete way the size of these virus particles—which are called virions. When you hold your thumb and index finger so close to each other that they are just barely not touching, the little space between them is about one millimeter (1 mm). A red blood cell is around seven one-thousandths of a millimeter in diameter (0.007 mm), and the diameter of the red blood cell is about 60 times larger than that of an influenza virion (0.00012 mm). Only the electron microscope and the image-making techniques associated with it made possible the visualization of virions.
Since then researchers have been able to create ever more detailed images of the fine structure of the virus body. Today we know, for example, that the rod-shaped tobacco mosaic virus has an outer protein shell that is helically twisted around a helix of RNA. Each type of virus has not only its particular life cycle but also a specific structure that can be made visible through an electron microscope. All types have in common a protein shell (capsid) on the outside surrounding DNA or RNA in the inside.
Viruses are typically viewed as “obligate parasites,” meaning that they must be within living cells in order to reproduce and do not have the possibility to reproduce on their own. They gain entry to a living cell and release their DNA or RNA into it. The cell responds by reconfiguring its own metabolism and produces the substances (proteins and nucleic acids) that are specific to the virus. The cell dies upon release of the newly formed virions, which in turn might infect other cells.
Image of tobacco mosaic virion (virus particle) created by coating virus material with a heavy metal to make structural features visible; magnified 160,000 times with electron microscope (public domain image).
Diagrammatic representation of the detailed structure of a part of the rod-shaped the tobacco mosaic virion. The coiled protein coat surrounds the coiled RNA core (public domain image)
Mutual Dependency Between Virus and Host Organism
Writing in 2000, Nobel prize-winning molecular biologist Joshua Lederberg argued that it is time to move beyond the war metaphor in regard to infectious diseases. He was deeply concerned about past epidemics and new ones that would come in the future. But nonetheless he spoke of overcoming what he called the Manichaean view of microbes—“We good; they evil.”10 He writes:
Perhaps one of the most important changes we can make is to supercede the 20th-century metaphor of war for describing the relationship between people and infectious agents. A more ecologically informed metaphor, which includes the germs’-eye view of infection, might be more fruitful. Consider that microbes occupy all of our body surfaces. Besides the disease-engendering colonizers of our skin, gut, and mucous membranes, we are host to a poorly cataloged ensemble of symbionts to which we pay scant attention. Yet they are equally part of the superorganism genome with which we engage the rest of the biosphere.
Lederberg made a plea for ecological view: “An axiomatic starting point for further progress is the simple recognition that humans, animals, plants, and microbes are cohabitants of the planet.” In reference to the AIDS epidemic he wrote that “our focus on extirpating the virus may have deflected less ambitious, though more pragmatic, aims, including learning to live with the virus by nurturing in equal measure the immune system that HIV erodes.” He would not be encouraged to hear that in 2018 around 770,000 people died of AIDS-related diseases.11
Since Lederberg wrote these words, much more research has been done, showing—for example in relation to the human microbiome—how intimately our lives are connected with the bacteria that dwell in our intestines and on our skin. In the past couple of decades researchers have taken into consideration the ecological nature of viruses as well.
An important first step for adequate understanding is to distinguish between the virus as an organized body—the virion—and the life cycle or doings of the virus within the host organism— the virus as process.12 Imaging techniques today lead us to picture viruses, and substances such as proteins or DNA, as solid, clearly bounded entities. The virion we picture is a static, solid entity devoid of all fluidity. As far as we know, virions are spread passively—via fluids, the air, or direct contact—from one organism to another. But as soon as the infection process begins, the presence of the virus in the organism comes to expression as activity; entering the host cell and replicating involve the virus as a doing. In its doings the virus is an ongoing process in which organized material states are being created and transformed.
The response of an organism to these doings brings about what we call the infectious disease. Infection is not a one-way street. It is an interaction between viral process and processes integral to the organism. For example, if a virus is to enter a cell, it needs to bind to a host organism receptor on the cell’s surface. In the case of coronaviruses—as far as is understood today—the virus’s spike protein binds the virus to a specific type of host cell receptor (ACE2). Then the spike protein is transformed by host enzymes so that the viral envelope and the cell’s membrane can fuse, allowing the virion to release its RNA into the cell. Which cells are infected is specific to both the virus and the host organism.
The replication of a virus in cells calls forth immune responses from the host organism. Symptoms of an infectious disease such as fever and inflammation are the body’s whole organism response to the virus and not the independent doings of the virus. A person’s body can “over-respond” to the infection and thereby stimulate the inflammatory process that destroys its own tissues, which in the case of COVID-19 can happen with the respiratory tissues of those individuals who become critically ill.
However, even the proliferation of virions within a person does not mean the person will become ill. With COVID-19, many people harbor reproducing viruses, but show no symptoms.13 The broad spectrum between no symptoms and extreme responses that can lead to death, shows how dependent the effects of infection are on the host environment. These great differences in the way people respond and interact with the virus present a great riddle.
It is surprising for most of us to learn that viruses are also inhabitants of our organisms throughout life—and not only when we have a viral illness. Today there are at least ten types of viruses that are continually present in cells of most people on earth and many more types that fewer people carry.14 In some instances, such as chicken pox, the virus is initially connected with the outbreak of an infectious disease and then resides in certain types of cells for the rest of life. With other types of viruses there are no symptoms at all (e.g. adeno-associated virus, anellovirus) or only occasionally in immunocompromised individuals (e.g. polyomaviruses). In the case of the asymptomatic annellovirus, it has been estimated that a billion virions are replaced daily. Human feces contain up to a billion virions of different types per gram.15
In a review of virus-host interactions, microbiologist Ken Cadwell concludes that the adverse or beneficial effects of viruses “are dependent on the anatomical location, host genotype, and the presence of other infectious agents and commensal microbes. It is often the context that determines whether a virus is deleterious, neutral or beneficial to the host.”16
To consider viruses as the causes of diseases is an oversimplification. As immunologist Herbert Virgin and colleagues point out, there is always a reciprocal relation between virus and host. For example, when people have genetic variations or mutations that predispose them to reacting to viruses that otherwise are rarely implicated in a disease, “in a real sense, the genes in the host “cause” the disease, as the viruses can infect many but cause disease in only a few.”17
Viral disease is a mutual interaction between the host organism and the virus. It would be much more fruitful to speak of the virus as a necessary condition for certain diseases, just as a predisposition in the host organism is a necessary condition. The use of the term “cause” in medicine, and in biology more generally, is misleading and would be best to avoid altogether. It suggests that the one specific entity is making something happen—“this causes that.” But in life, what people call the cause is always embedded in a context, and in interplay with this context the effect arises. In life there are only reciprocal relations.
Increasingly scientists have been discovering in bacteria, fungi, plants, animals, and humans viruses that are helpful rather than harmful.18 For example, among the viruses that infect bacteria, called bacteriophages, there are some that adhere to the mucus layer in the gut and then infect and destroy pathogenic bacteria. Bacteriophages in our gut form the bulk of viruses that humans harbor, and they differ from individual to individual.
Bacteriophages can also transfer genes from one bacterium to another, which is one way that antibiotic resistance spreads in bacteria.19 Horizontal gene transfer is also widespread in oceans. There is a remarkable abundance and diversity of viruses in the ocean; most of them, as far as is known today, are phages that infect bacteria. While much is still to be understood, it is clear that these viruses alter the bacterial and microbial community near the surface of the water, and when the bacteria they have infected die, nutrients are released and influence nutrient cycling in the ocean.20
Molecular biologists have discovered segments of DNA in the genomes of all organisms that evidently have a viral origin (as gleaned from the specifics of the DNA sequence). These endogenous viral elements (often called retroviruses) have become incorporated into the genome of an organism. It is estimated that eight to nine percent of our genome consists of such viral elements.21 They are also present in plants and animals and can have a variety of functions. In mammals, for example, one such retrovirus plays an important role in the normal development of the placenta.22 Horizontal gene transfer and retroviruses show how it is impossible to speak of clear and fast boundaries between different kingdoms of life. Bacteria and viruses are normal constituents of all organisms.
There are fascinating cases of symbiosis involving a variety of different organisms and viruses. Here are two examples.23 A particular grass species (Dichanthelium lanuginosum) can grow in soil temperatures greater than 50 degrees C (122 degrees F). The grass houses a fungus within it. Neither the grass nor the fungus can grow in these extreme conditions alone; the symbiotic relation is needed. Moreover, it was discovered that the fungus harbors a virus, without which the fungus-plant symbiosis loses its heat tolerance. So it is a three-member symbiosis that allows a plant—and its partners—to thrive in such extreme conditions.
In a similar way, aphids harbor a number of different symbiotic bacteria. In the pea aphid a particular bacterial species protects the aphid against a parasitic wasp. The wasp lays eggs in the aphid and the bacteria create a toxin that kills the developing wasp larvae. Further investigation showed that DNA from the virus is required for the bacteria to produce the toxin.24
Just as more and more instances of the mutual beneficial relations between bacteria and other organisms have been discovered in the past decades, it seems likely that scientists will increasingly discover how viruses often play a multifaceted role in the large web of life processes on earth.
A More Expansive Perspective
In the course of the past decades scientists have discovered how bacteria and viruses are much more than just enemies. They and all other life forms interact with each other and inhabit each other. There is a great flux and fluidity in the exchange and transformation of substances, including genetic material. And nonetheless there is an integrity to each of the myriad different species of organisms on the planet—each forms and reforms itself in constant interaction with other organisms and influences in the environment. But every “itself” is itself by virtue of others. “Otherness” and “separateness” are, therefore, a matter of degree. Neither can be conceived of as being absolute.
When we take this insight seriously, we find it opens new perspectives for understanding life on earth. We can consider the earth as one large and unitary sphere of living existence that differentiates into myriad kinds of activities and presences that remain related to one another. How they relate and interact is continually changing and evolving. Yet there is always connectedness or interweaving relatedness.
This perspective opens up an array of questions and ways of approaching and understanding phenomena. Most broadly, when we leave behind the paradigm that the world is inhabited by separate entities that interact—whether through competition or cooperation—to form a collective, we can ask: How are individual events embedded in and revelatory of the larger whole? In a given species of plant or animal, all the parts and processes are informed by the whole and expressive of it. So we can also inquire more broadly: How is a particular event or phenomenon expressive of the larger whole of which it is a part? We are not looking for causality, but for the meanings that may express themselves in the various relations we encounter.
When we begin to see how viruses are part of the dynamic web of life, then we can move beyond entrenched and one-sided pictures of the enemy, the good and the bad, the us and the them. This by no means makes us blind to the great harm that can occur through viral infections. But then the question is not only how to gain victory over the enemy. Beyond all the important immediate measures to help people stay safe and regain health during an epidemic or pandemic, larger questions arise:
What are the different facets of virus-host interactions in disease and in health? What are the characteristics of earth ecology, societal relations, and the ways we think, feel, and act that make possible and come to expression in the COVID-19 pandemic? What underlying imbalances in our organisms, in our souls, and in our societies provide the conditions for the pandemic to occur in the way it has? We human beings are the ones, different from all other organisms on earth, who intentionally shape and re-shape the earth. No doubt, the current situation has something to teach us.
Notes and References
These stimulating conversations were held among Henrike Holdrege, Jon McAlice, Sergio Spalter, MD, and myself. I would like to thank them all for the productive thinking together, and also for their comments on the various drafts of the paper.
Mayer, A. 1886. Über die Mosaikkrankheit des Tabaks. Die Landwirtschaftlichen Versuchs-Stationen, vol. 32, pp. 451–67. (Translated and reprinted in 1942 as “Concerning the Mosaic Disease of Tobacco,” Phytopathological Classics vol. 7, pp. 11–24).
Quoted in: Lustig, A. and A. J. Levine. 1992. One Hundred Years of Virology. Journal of Virology vol. 66, pp. 4629–31.
Beijerinck, M. W. 1898. Über ein Contagium Vivum Fluidum als Ursache der Fleckenkrankheit der Tabakblätter. Verh. Kon. Akad. Wetensch. vol. 63, pp. 3–21. (Translated and reprinted in 1942 as “Concerning a Contagium Vivum Fluidum as Cause of the Spot Disease of Tobacco Leaves,” Phytopathological Classics vol. 7, pp. 33–52.)
Bos, L. 1999. Beijerinck’s Work on Tobacco Mosaic Virus: Historical Context and Legacy. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, vol. 354, pp. 675–85.
Sankaran, N. 2018. On the Historical Significance of Beijerinck and His Contagium Vivum Fluidum for Modern Virology. History and Philosophy of the Life Sciences vol 40. https://doi.org/10.1007/s40656-018-0206-1
Schadewaldt, H. 1975. Die Entdeckung des Virus der Maul- und Klauenseuche. Deutsche medizinische Wochenschrift vol. 100, pp. 2355–59.
Stanley, W. M. 1935. Isolation of a Crystalline Protein Possessing the Properties of Tobacco Mosaic Virus. Science vol. 81, pp. 644–45.
Van Helvoort, T. 1994. History of Virus Research in the Twentieth Century: The Problem of Conceptual Continuity. History of Science vol. 32, pp. 185–235.
Kausche, G. A., Ruska, H. 1939. Die Sichtbarmachung der Adsorption von Metalkolloiden an Eiweisskörper. Kolloid-Zeitschrift vol. 89, pp. 21–26. https://doi.org/10.1007/BF01518802
Lederberg, Joshua. 2000. Infectious History. Science vol. 288, pp. 287–93. https://doi.org/10.1126/science.288.5464.287
Dupré, John and Stephan Guttinger. 2016. Viruses as Living Processes. Studies in History and Philosophy of Biological and Biomedical Sciences, vol. 59, pp. 109–16.
See, for example:
Day, Michael. 2000. Covid-19: Four Fifths of Cases are Asymptomatic, China Figures Indicate. BMJ vol. 369. https://doi.org/10.1136/bmj.m1375
Mizumoto, Kenji et al.2020. Estimating the Asymptomatic Proportion of Coronavirus Disease 2019 (COVID-19) Cases on Board the Diamond Princess Cruise Ship, Yokohama, Japan, 2020. Euro Surveillance vol. 25(10): pii=2000180. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180
Nishiura, Hiroshi et al. 2020. Estimation of the Asymptomatic Ratio of Novel Coronavirus Infections (COVID-19). International Journal of Infectious Diseases. https://doi.org/10.1016/j.ijid.2020.03.020
Virgin, Herbert W. et al. 2009. Redefining Chronic Viral Infection. Cell vol.138, pp. 30–50. https://doi.org/10.1016/j.cell.2009.06.036
Virgin, Herbert W. 2014. The Virome in Mammalian Physiology and Disease. Cell vol. 157, pp. 142–150. https://doi.org/10.1016/j.cell.2014.02.032
Cadwell, Ken. 2015. The Virome in Host Health and Disease. Immunity vol. 19, pp. 805–13. https://doi.org/10.1016/j.immuni.2015.05.003
Virgin, Herbert W. et al. 2009. Redefining Chronic Viral Infection. Cell vol.138, pp. 30–50. https://doi.org/10.1016/j.cell.2009.06.036
Pradeu, Thomas. 2016. Mutualistic Viruses and the Heteronomy of Life. Studies in History and Philosophy of Biological and Biomedical Sciences vol. 59, pp. 80–88. https://doi.org/10.1016/j.shpsc.2016.02.007
Roossinck, Marilyn J. 2011. The Good Viruses: Viral Mutualistic Symbioses. Nature Reviews Microbiology vol. 9, pp. 99–108. https://doi.org/10.1038/nrmicro2491
Davidson, Alan R. 2018. A Common Trick for Transferring Bacterial DNA. Science vol. 362, pp. 152–3. https://doi.org/10.1126/science.aav1723
Breitbart, Mya. 2012. Marine Viruses: Truth or Dare. Annual Review of Marine Science vol. 4, pp. 425–48. https://doi.org/10.1146/annurev-marine-120709-142805
Rohwer, Forest and Rebecca Vega Thurber. 2009. Viruses Manipulate the Marine Environment. Nature vol. 459, pp. 207–12. https://doi.org/10.1038/nature08060
Virgin, Herbert W. et al. 2009. Redefining Chronic Viral Infection. Cell vol.138, pp. 30–50. https://doi.org/10.1016/j.cell.2009.06.036
Pradeu, Thomas. 2016. Mutualistic Viruses and the Heteronomy of Life. Studies in History and Philosophy of Biological and Biomedical Sciences vol.59, pp. 80–88. https://doi.org/10.1016/j.shpsc.2016.02.007
Roossinck, Marilyn J. 2011. The Good Viruses: Viral Mutualistic Symbioses. Nature Reviews Microbiology vol. 9, pp. 99–108. https://doi.org/10.1038/nrmicro2491
Moran, Nancy A. et al. 2005. The Players in a Mutualistic Symbiosis: Insects, Bacteria, Viruses, and Virulence Genes. PNAS vol. 106, pp. 16919–26. https://doi.org/10.1073/pnas.0507029102