The Story of an Organism: Common Milkweed
Craig Holdrege
View article as PDF | Listen to an abridged version of the article
“All I am saying is that there is also drama in every bush, if you can see it. When enough men know this, we need fear no indifference to the welfare of bushes, or birds, or soil, or trees. We shall then have no need of the word ‘conservation,’ for we shall have the thing itself.” — Aldo Leopold (1999, p. 172)
I had casually observed common milkweed (Asclepias syriaca, Asclepiadaceae) but never paid too much attention to it. True, I was fascinated by its big globes of flowers and, in the fall, by its beautiful seeds that floated through the air on their tufts of white silk. I also knew that common milkweed is the main food plant for monarch butterfly larvae. But it was only when I was preparing for the 2006 summer course at The Nature Institute and when I noticed the flowers of common milkweed beginning to open, that I looked closely at them for the first time. I realized that the plant has a highly complex flower structure and, in addition, observed how the flowers were being visited by many different insects. Milkweed had finally caught my attention, and I decided that we should focus on it for our initial plant study in that weeklong course.
This study proved to be particularly intense. Milkweed drew us all into its world of refined structures. It took us a good while just to get clear about the flower parts and their relation to more “normal” flowers. (There were a number of trained biologists in the course.) We also observed interaction with insects and saw how flies sometimes became caught in the flowers and died. After four morning sessions dedicated to milkweed, we had made an acquaintance with the plant. And we had begun to get a glimpse of its unique characteristics, which became all the more apparent through a comparison with St. John’s Wort (Hypericum perforatum), which was also flowering at the time. During these days we had begun to see milkweed as a remarkable composition expressing itself, on the one hand, in robust structures such as the rhizomes, upright stems, and leaves and, on the other hand, in the high-grade refinement of the flower. This glimpse of the nature of common milkweed initiated my journey to get to know the plant better. It was a wonderful gift to have a study initiated by a group effort during a course.
I began studying the plant’s life history from first emergence in spring to opening of the fruits and seed dispersal in October and observed and compared plants in different patches (colonies). I collected and then planted seeds from plants from different colonies, and observed germination and young seedling growth. I drew and photographed plants and also pressed the leaves of numerous plants. Finally, I observed the insects that associate with common milkweed. In addition to my own observations, which continued over a couple of years, I carried out an extensive review of the scientific literature on common milkweed.
My attempt here is to give an initial portrayal of common milkweed — its life history and relations to some of the organisms with which its life is bound up. I integrate my own observations with information taken from the scientific literature. This literature is a reflection of the countless hours researchers have spent observing common milkweed and its ecology, designing and carrying out experiments, and interpreting their findings. In whole-organism study the task is to bring together the observations of many researchers and to paint a coherent picture of the plant and its relationships. In Goethe’s words, the goal is “to portray rather than explain” (1995, p. 57). Such a portrayal is a kind of story of the plant. To craft such a portrayal, it is necessary to omit many of the hypotheses and interpretations that presently guide most scientific research on the plant. Such hypotheses want to explain why, for example, milkweed has its specific flower form, why it secretes so much nectar, or why it contains in its leaves and stems a sticky, toxic sap. My question is not “why?” but rather “how?” I want to see how milkweed is formed, how its parts relate to each other, and how it relates to its environment. I hope that this portrayal will provide a vivid picture of a remarkable plant and at the same time exemplify a process that can be applied to other organisms as well.
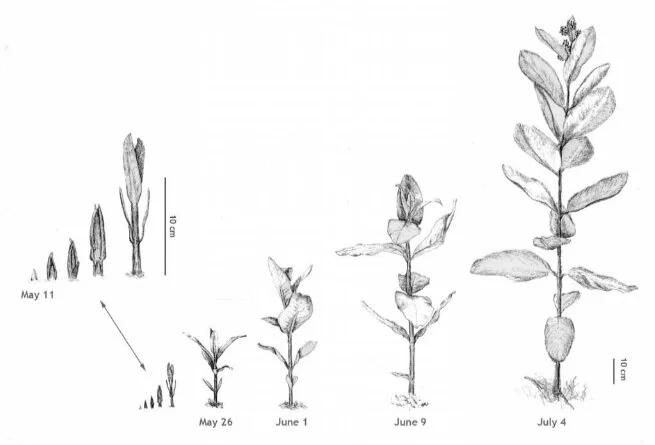
Figure 1. Shoot development in common milkweed (Asclepias syriaca). Shoots from one colony, drawn to scale. Summer 2007.
Vegetative Development
It is early May in upstate New York. When you look at roadsides and old fields, there is still much of the past to be seen — the dead, brown and gray remnants of last year’s growth. The dry, brittle leaves of some grasses and wildflowers, and the crisscross of matted stems from asters, goldenrods and milkweed provide the immediate surroundings for this year’s fresh green emerging vegetation. The shoots of grasses, goldenrods, and other wildflowers are rising out of the soil; common milkweed shoots emerge only later. You have to get down close to the ground to see the stout little green and brown-reddish spears growing through the leaf and stem litter (see Figure 1).
Figure 2. Rhizome of a common milkweed (Asclepias syriaca) laid bare during the late spring. Top: branching rhizome. Bottom: close-up. a: rhizome; b: roots; c: shoot from previous year; d: buds that were formed in the summer/fall, some of which will unfold in the following spring; e: scars from the base of a shoot from the previous year. (For the scale, see dime in the lower right-hand corner of the top photo.)
And where you find one shoot emerging, you usually find many. Common milkweed grows in colonies that tend to get more populated each year, unless something in the environment inhibits their exuberant growth. There can be hundreds or thousands of shoots, depending on the size of the colony. All these little shoots grow from buds that have overwintered; they developed the previous summer on rhizomes (underground stems) and remain dormant until the following spring. The rhizome grows and branches each year, never showing itself above ground and forming an extensive network out of which the many individual shoots grow (Figure 2).
A colony is thus one large plant. You could compare it with an individual tree, but instead of growing a woody trunk that extends upward and branches outward to create a lasting form, common milkweed branches underground and some old parts of the rhizome die away while new ones arise. So, botanically speaking, a colony is a clone — the individual shoots are genetically identical, having originated from a single seed through vegetative growth. So when I observe what looks like an individual specimen of common milkweed, the shoot I am observing is a one side-branch of a much larger plant, namely the whole colony.
One perceptual hint of this larger unity is that milkweed colonies are usually quite uniform and differ from other colonies in characteristic ways. One colony might exhibit shoots that are long, narrow and distinctly tinged red; another colony may have more pointed-tipped leaves; a third may have especially deep-pink-colored flowers. Such characteristics are hereditarily anchored and therefore are common to all parts of the colony.
Back to the emerging shoots. I will focus now on one shoot and its development, but we should keep in mind that what I’m describing is occurring with many other shoots within the colony at more or less the same time. As a shoot grows upward, the first small lance-shaped leaves begin to unfold. The leaves are arranged in an opposite pattern, meaning that two leaves emerge at the same height out of the stem, opposite each other. The next pair of leaves is offset by about ninety degrees so that a distinct leaf arrangement (phyllotaxis) emerges (Figure 1).
The stem is stout and grows quite straight upward. It is significantly thicker than its counterpart in asters or goldenrod, but not so dense. While the first leaf pairs near the ground are small, the leaves soon became long and broad. Milkweed has much bigger leaves than most other plants growing in the roadside/old field community. The leaves have a short thick petiole that becomes the distinct midrib of the leaf blade. The midrib branches off into fairly straight, diagonally ordered side veins that are clearly visible on the leaf underside. The oblong leaves have a smooth, clear margin (Figure 3).
Figure 3. Pressed leaves from one shoot of common milkweed (Asclepias syriaca). The first leaves are at the bottom, the last leaves are at the top. When the plant is full in flower, the lower pairs of leaves have died and fallen off the plant. The lower pairs were picked and pressed shortly before they had wilted.
Over the course of May and through June the shoot grows vigorously, reaching a height of about 1 to 1.5 meters. It surpasses in length the goldenrods that had begun their development a few weeks before the milkweed. As the shoot extends, new leaf pairs unfold, each pair larger than its predecessor. After a shoot has developed about seven to nine pairs of leaves, the first flower buds become visible among the still unfolding uppermost leaves. The stout little stems (peduncles) that carry the groups of flower buds do not grow out of leaf axils, as is the case in most flowering plants. Rather, in all but one species of the genus Asclepias, the flowers grow out of the main shoot slightly to the side of a leaf (Woodson, 1954). Interestingly, where flowers develop, the leaf arrangement also changes. Subsequent leaf pairs are no longer opposite to each other, but shift to an angle of about 120°. Also — and this typifies most flowering plants — the leaves become smaller in the flowering region. The uppermost leaves contract to a size comparable to the very first leaves on the shoot, but tend to be more pointed and elliptical in shape. These changes in vegetative structure point us to the next developmental wave in milkweed’s life history — the flower.
From Flower to Fruit
Among the unfolding upper leaves you can see small grayish-green balls of tightly grouped flower buds (Figure 4). As the buds grow and gradually turn pink, the stout stem that carries them away from the main shoot extends diagonally upward. Each flower bud has, in turn, its own delicate stalk, which also lengthens, and the tight ball becomes a looser and larger sphere.
Figure 4. Unfolding umbel of flowers in common milkweed (Asclepias syriaca). For context within the whole shoot, see Figure 5.
All the individual flower stalks (pedicels) originate at the apex of their common stem, which means that in botanical terms the milkweed inflorescence is an umbel. There can be anywhere between 10 to nearly 200 flowers in one umbel; the average flower number per umbel has been reported as 76 (Willson & Rathcke, 1974) and 104 (Kephart, 1987). While in many umbel-forming plants, as in most members of the carrot family, the flowers spread out into a plane to form a disk, the umbel of the common milkweed maintains its spherical form until the flowers wilt.
Figure 5. One shoot from a colony of common milkweeds (Asclepias syriaca) shown over the course of three weeks during its flowering phase. On June 22 no flowers are open yet and one sees that the lowermost inflorescence on the plant is most developed. After a week (July 1) all the flowers of the lowermost inflorescence are wilting while those in the uppermost inflorescence have yet to open. On July 7 most flowers have wilted. The flower remnants have dropped off the plant or are completely shriveled by July 13; only a few fruits develop from the approximately 150 flowers.
As Figure 5 shows, the lowermost umbel on a given shoot opens first, then the one next higher up, and so forth. A shoot has an average of 3 to 6 umbels, but some shoots have none and others up to 10 (Hartman, 1977; Willson & Rathcke, 1974). Usually when the lowermost umbel is already wilting, the uppermost one is still in bloom. Milkweed flowers are long-lived in comparison to the flowers of other plants, since any single flower can be open for over a week (Kephart, 1987, gives an average of 9 days; Wyatt & Broyles, 1994, an average of 4 to 8 days). The whole phase of flowering in a colony lasts about 4 weeks.
Long before you come close enough to a colony to be able to see its flowers, you can smell that it is in full bloom. The sweet scent of the nectar-filled flowers carries far. As you approach the colony you see the rich-pink flowering spheres and a multitude of insects crawling and flying around. Honey bees, native bees such as bumbles bees, ants, and a variety of butterflies move from flower to flower, umbel to umbel, drinking nectar from the blossoms. Along the way they pollinate the flowers, a strangely intricate process related to the complex anatomy of milkweed flowers that I will discuss below.
With the multitude of flowers and pollinators, you would think that many fruits (pods) would develop, but this is not so. Although one shoot may have between 300 and 500 flowers, only few develop into pods — according to Willson and Rathcke (1974) about one pod for every 150 flowers, while Hartman (1977) found that one pod developed for every 60 to 100 flowers. Most flowers in an umbel wilt, while the occasional fruit shows itself through stalk thickening and the white and fuzzy swelling that is the developing pod (Figure 6, A-D).
Figure 6-A. First seeds of the developing pod.
Figure 6-B. The larger pods lower down are further developed, while the pods from the upper inflorescence are just beginning to form.
Figure 6-C. Later in the summer, a stem with pods at a variety of stages of development.
Figure 6-D. Full-sized pods (about 10 cm long); note the upright orientation.
While vegetative growth is rapid and expansive, and flowering is a period of bursting productivity, pod development is slow and extended. During July, August, and September the pods grow and in their inner cavity the seeds develop. One interesting feature of pod development is that the stalk that carries the pod — regardless of the angle at which it originally extends from the umbel’s stalk — twists and curves into a position such that the pod becomes oriented vertically. The pod expands slightly laterally, but mainly grows in length. By October or early November the pods have reached their full size and maturity. The suture along the convex side of the pod splits open and the neatly ordered, tightly packed seeds become visible (Figure 7, A-D). It looks as if an artist had laid the seeds out. With further opening of the pod, the seeds begin to fall and float away. Each seed has lovely white silky extensions (comas) that allow them to be carried by a breeze — even though, as seeds go, common milkweed seeds are significantly larger and heavier that those of other old-field species (Wilbur, 1976). Interestingly, Kephart (1987) found that although individual shoots within a colony may flower three weeks later than others, the fruits in that colony release their seeds simultaneously.
Figure 7-A. Milkweed pod splits.
Figure 7-B. Pod opens, seeds intact.
Figure 7-C. Seed release begins.
Figure 7-D. Wind releases more seeds; pod empties.
I mentioned above the paucity of pods in comparison to the wealth of flowers in a milkweed shoot or colony. But each pod is full of many seeds — one study gives an average of 226 (Willson & Rathcke, 1974) — so that in a colony of, say, 1,000 shoots, hundreds of thousands of seeds will spread into the environment. You would, as a result, expect to easily find seedlings of milkweed plants in areas around existing colonies. As I began to study common milkweed I searched for pictures of seedlings so I could identify them. To my surprise I could find none, although you can find images of the seedlings of most agricultural field weeds. I have found no studies of milkweed that describe seedlings in the wild. Evidently, milkweed seeds are not prolific germinators.
Since, however, new and young colonies can be observed, at least once in a while seeds must germinate and some seedlings take hold. As field experiments show (Agrawal, 2005), already in the second year a plant can produce multiple stems, some over a meter apart from each other, showing the vigorous growth of the rhizome.
In this sketch of its life history we can see how, up through flowering, common milkweed is characterized by exuberant vitality: vigorous underground rhizome growth; yearly expansion of many long, large-leafed shoots; production of numerous flowers that secrete copious amounts of nectar. After flowering, milkweed pulls back and concentrates its vitality into formation of a relatively small number of pods, but each swells into a large pod that houses a multitude of apparently viable seeds that spread into the larger environs. Yet only a few of them form new colonies.
Flower Morphology and Pollination
While the form of the leaves in common milkweed — and other members of the genus as well — is simple, and the shape hardly transforms from bottom to top of the stem, the flowers are highly complex and differentiated. It took much detailed botanical study to identify and relate milkweed flower structure to the anatomy of other flowers (Bookman, 1981; Woodson 1954).
Figure 8-A. Flower buds still closed except for one partially open in the lower left hand corner.
Figure 8-B. Two sepals and two petals have folded back.
Figure 8-C. An open flower, the petals are not yet fully folded back onto the stalk.
Figure 8-D. Side view of a fully opened flower.
When the flower opens, five small green sepals fold back and then five larger elliptical white to pink petals also fold back and come to lie more or less parallel with the flower stalk (Figure 8, A-D). In most “typical” flowers of other kinds of plants, you find a circle of petals surrounding a circle of stamens, whose anthers release pollen. In the center of the flower is the pistil in which the seeds will develop. Neither the stamens nor the pistil are clearly recognizable in the milkweed flower. Rather, a number of accessory organs form out of the partially fused stamens and pistil.
Figure 9-A. Side view of flower.
Figure 9-B. Corona with two visible pollinia.
Figure 9-C. Corona from above.
Figure 9-D. Flower structure of common milkweed (Asclepias syriaca). See text below for description.
Figure 9-D gives a diagrammatic representation of the milkweed flower. Above the petals is a so-called corona consisting of five cup-like hoods out of which extend little curved horns. The hoods hold the nectar that attracts so many insects on warm, sunny summer days. In between the hoods are little vertical slits. Each slit opens into a stigmatic chamber. (What it has to do with the stigma, we will see further below.) Above the slit there is a tiny black knob, the corpusculum. What one doesn’t see is that the corpusculum has two little arms (called translator arms) that extend into the two upper sides of the chamber. Each arm attaches to a golden package of pollen, called a pollinium. Each of the pollinia houses hundreds of pollen grains.
Unlike the pollen in most flowers, which is released from the anthers while they are still attached to the flower, in milkweeds the pollen remains contained within the pollinia until it comes in contact with the stigma of a flower. Only orchids — also plants with complex flowers — package their pollen in a similar way.
The only way for the pollinia to escape their chambers is via insects. As already mentioned, milkweed flowers are visited by a multitude of insects that are in search of nectar — which they find in generous supply. Not only is the nectar very rich in sugar content, being up to 3% sucrose, but the supply is also renewed over the life of the individual flower (Southwick, 1983; Wyatt, Broyles, & Derda, 1992). Milkweed flowers produce much more nectar than the many insects feeding on it could ever remove.
While insects are moving around on a flower — they sometimes go from hood to hood on a single flower — one of their legs may slip into a slit. I have often observed honeybees (Apis mellifera) and flies struggling to pull a leg out of the slit. Most often it succeeds by pulling its leg upward whereby the leg hooks into a groove on the corpusculum at the top of the slit and as a result the insect pulls the whole pollinarium — corpusculum, two translator arms and two pollinia — out of the stigmatic chamber. One study (Morse, 1982) showed that a bumble bee picks up on average a new pollinarium every 2 to 5 hours and that the same pollinarium remained attached to the legs for on average 2.5 hours, while if attached to the mouthparts, the pollinarium stayed attached for 10 hours. As an insect continues its nectar foraging, it often accumulates multiple pollinaria. One pair of researchers (Jennersten & Morse, 1991) reported finding up to 35 pollinaria on a single insect; sometimes they are hooked together in chains of ten or more dangling from an insect’s leg. Most often one sees honeybees with many pollinaria attached to them (Figure 10, A-B). (In Figure 9-B, pollinia can be seen outside the stigmatic chambers; in such cases the pollinarium detaches from the insect’s leg before it is completely removed from the stigmatic chamber.)
Figure 10-A. A honeybee moves through an umbel of milkweed flowers.
Figure 10-B. Close-up of a honeybee on a milkweed flower with multiple pollinia dangling from its legs. (Note the slit and corpusculum (dark spot) between two of the hoods of the flower in the lower left of photo B).
Smaller insects — I have observed mainly flies — may be unsuccessful in removing a leg from a slit and the limb will tear off in the process. I have also seen dead flies hanging from a flower with a leg still caught in a slit. Larger pollinators like bumble bees and honeybees are more likely to extract pollinaria without losing a body part.
What happens to the pollinaria? Many will simply drop away or be rubbed off as the insect moves around. A few will find their destination in another flower. And just as the removal of the pollinaria is both a haphazard and narrowly constrained process, so also is pollination. First, something remarkable and absolutely essential for pollination occurs with the pollinarium itself. After an insect has been carrying around a pollinarium for about ninety seconds, the pollinarium dries out, and in the process the translator arms rotate ninety degrees (Wyatt & Broyles, 1994). This torsion is highly significant because it brings the pollinium into a position that allows it to slip into a slit when an insect moves over one. Before the torsion, the broad side of the pollinium usually faces the slit as the insect crawls along; afterwards the narrow side of the pollinium is in line with the slit so that if the insect is moving at just the right height over a slit, the pollinium can slide into the stigmatic chamber. As the insect moves ahead, the translator arm breaks and the pollinium remains behind. Once in the chamber, one edge of the pollinium rests against the receptive wall of the stigmatic chamber. While in most flowering plants the stigma is open to direct contact with the air and visiting insects, the five receptive stigma surfaces in milkweeds are enclosed within five stigmatic chambers, open to the world only through the narrow slit. So in milkweed flowers, both pollen grains and stigmatic surfaces are housed in enclosed structures.
Only when the pollinium slides into the stigmatic chamber can this encapsulation be overcome. Everything I will now describe is visible only when one dissects flowers at different stages of the pollination process and examines the structures under a microscope (Sage & Williams, 1995). The stigmatic chamber is the source of nectar for the hoods, and when the pollinium is inserted into the chamber, it is bathed in nectar and begins to swell. Within a few hours the edge that is in contact with the receptive inner surface of the stigmatic chamber breaks open and multiple pollen tubes grow out of the pollen grains. The tubes grow down the style and into one of the two ovaries of a flower. An ovary contains about 200 ovules (Baum, 1948) and each one needs to be fertilized by the nucleus from one pollen tube. Interestingly, when fertilization occurs, virtually all the ovules are fertilized—one rarely finds milkweed pods with just a few seeds (Wyatt & Broyles, 1994).
One could at first think that the highly specialized pollination apparatus, which provides a seemingly perfect fit between pollinium and stigma, would guarantee successful fruit set. But this is not the case. In the first place, the specialization also means that relatively few pollinia actually are successfully inserted into a stigmatic chamber. Secondly, researchers discovered that common milkweed is self-incompatible (reviewed in Wyatt & Broyles, 1994). This means that the pollinium from a flower that is inserted into a flower of the same colony will normally not bear fruit. The pollen tubes grow into the ovary, but the seeds do not develop. Because insects are moving largely within a given colony, it is likely that most of the pollinia inserted come from flowers of the same colony and will therefore not lead to successful fruit set. One study (Pleasants, 1991) using radioactively labeled pollinia found that a third of the pollinia inserted were from the same umbel and most of the other inserted pollinia came from the same colony; only a few were carried to other colonies and successfully inserted.
If this “inefficiency” — we could also call it over-abundance — were not odd enough, even when researchers cross-pollinated flowers of related Asclepias species by hand, fruit set never exceeded 20% and was usually considerably lower (Wyatt & Broyles, 1994). Thus, overall, the specialization of milkweed pollination, at least in relation to successful fruit formation, is connected with a low vitality. If one looks, however, at a milkweed colony from the perspective of the many insects that feed on the flowers’ nectar, milkweed is contributing significantly to the vitality of those animals. Of course, the picture is more complex if we think of the occasional death of mainly smaller insects unable to remove a limb that has been caught in a slit.
One interesting feature of the relation between flower structure and insects is that, although all milkweeds have such specialized flowers, they are visited by and can be pollinated by a wide array of insects. The nectar also attracts ants and small beetles that usually contribute little toward pollination, but reap the benefits of the ample nectar supply (Fritz & Morse, 1981).
Abundant Animal Life
We have already seen that common milkweed is an important part of the life of insects that feed on its nectar. Observing nectar feeders on common milkweed, Southwick (1983) identified representatives from 15 different orders of insects (and one hummingbird species). Nectar was taken mainly during the day but also during the night by a variety of nocturnal moths. But these nectar-feeding insects represent only a minority of the insects and other arthropods that interact with milkweed. In the late 1970s Dailey and his colleagues carried out surveys of bugs (Hemiptera) and beetles (Coleoptera) found on common milkweed (Dailey, Graves, & Herring, 1978; Dailey, Graves, & Kingsolver, 1978). Over the course of ninety days, they found 132 different species of beetles, 18 of which they considered common visitors, since they collected more than 50 specimens of each of these species. They collected 45 species of bugs, 5 of which were common visitors according to the same criterion. Milkweed teems with insect life.
For many insects, milkweed is certainly a small and transient part of their habitat—or speaking functionally, a minor part of their ecological niche. They may nibble on the leaves and flower buds, or drink some nectar and then move on to other plants. As predators they may, like the bug Phymata fasciata, hide in the thicket of milkweed stems, leaves, and flowers, waiting for their prey of flies and small wild bees. And then there are the milkweed specialists, which I will discuss below, that feed almost exclusively on milkweeds. So milkweed provides food and a microhabitat for a multitude of organisms. Its exuberant growth — in rhizomes, stems, leaves, flowers, fruits, and seeds — allows abundant insect life to orient around it.
The Extended Organism
One striking feature of the common milkweed is that the entire life cycle of a number of insect species is tightly interwoven with it. There are at least 10 species of insects that feed only on common milkweed or other closely related milkweeds in the genus Asclepias (Agrawal, 2005; Price & Wilson, 1979; see Table 1 and Figure 11 A-G). These specialist species feed on milkweed rhizomes, shoots, leaves, flowers, or seeds. The most well-known of these is the monarch butterfly (Danaus plexippus). The adult butterfly lays its eggs on the leaves of common milkweed, the larvae live from its leaves and the milky sap the plants contain, and the adults drink from the flower nectar, although adults are not restricted to milkweeds.
Table 1. Milkweed-specific herbivores.
What is fascinating about the monarch and some of the other milkweed specialists is that they do not just feed on the plants, digest the substances, and then build up their own body substances. Rather, they store some of the components of the milkweed sap in their body. When a milkweed stem or leaf is damaged, it exudes a white sap. All you have to do is to scratch the stem with your finger nail and the white sap oozes out and streams down the stem until it gradually hardens. When, for example, a monarch larva bites into a leaf vein or stalk, the sticky (latex-containing) milky sap seeps out and the larva ingests it. It draws out of the sap a particular group of substances known as cardiac glycosides (cardenolides), and instead of breaking them down or excreting them, it stores them in its tissues. The concentration of cardiac glycosides in the tissues of a monarch is substantially higher than it is in the tissues of common milkweed. Interestingly, it is not only the larva that sequesters these substances; they are also retained in the adult, which has gone through the complete metamorphosis from caterpillar to butterfly. So part of the milkweed becomes an essential part of its insect predators.
Cardiac glycosides are bitter tasting and can disrupt the ionic balance of a number of different cell types in animals, including heart muscle, vascular smooth muscle, neurons, and kidney tubules (Malcolm, 1995). In high doses they can be fatal to an animal, but in nature this will rarely happen, since they cause vomiting in pre-lethal doses. We would imagine that common milkweed is protected against herbivores by the cardiac glycosides in its sap. Clearly, however, the sap does not prevent specialist herbivores from feeding on milkweed and sequestering cardiac glycosides, although some of these specialists avoid taking in large amounts of sap while feeding. The monarch and red milkweed beetle are known to bite into a milkweed leaf vein near the base of the leaf, which then exudes sap that flows back out of the more distally-located veins (Dussourd, 1999; Helmus & Dussourd, 2005). The insect then crawls to the periphery of the leaf and begins to feed from the part of the leaf that now contains little sap.
Unsurprisingly, researchers believed that by sequestering cardiac glycosides, milkweed predators may be protected against their own predators. Beginning in the 1960s researchers began testing this hypothesis and, as Malcolm (1995) concludes in a review, “much evidence is published to show that many prey species are well defended against predators by the presence of cardenolides” (p. 101).
Figure 11-A. Monarch caterpillar on milkweed leaf. (Danaus plexippus)
Figure 11-B. Monarch butterfly (Danaus plexippus) on milkweed.
Figure 11-C. Red milkweed beetle (Tetraopes tetraophthalmus)
Figure 11-D. Milkweed leaf beetle (Labidomera clivicollis).
Figure 11-E. Nymph of large milkweed bug (Oncopeltus fasiatus).
Figure 11-F. Adult large milkweed bug (Oncopeltus fasiatus).
Figure 11-G. Milkweed tussock larvae (Euchaetes egle).
So milkweed is helping those insects that prey on it become better protected from their own predators. This is, in a sense, a paradoxical situation in which a plant is providing protection for its predators, which increases the likelihood that there will be more predators to feed on it (Malcolm 1995). Theoretically, one could think that these specialists might eradicate milkweed. But neither the scientific literature nor my own observations indicate that milkweed populations are significantly harmed by the specialist herbivores associated with them. And it is not as if the monarch or other milkweed specialists have no predators. Prysby (2004) points out that both monarch adults and larvae are preyed upon at least occasionally by some birds, mice, ants, dragonflies, and wasps, and that the larvae can be parasitized by flies and wasps.
Most of the milkweed specialists that sequester cardiac glycosides are brightly colored (Figure 11). (Within a Darwinian framework, one interprets such coloring as warning coloration, also called aposematic coloration. The theory is that the bright colors and patterns evolved as a warning sign to predators that signals “keep off.”) Hartman (1977) noticed that most brightly-colored, cardiac glycoside-storing herbivores tend to move around the plant a good deal when feeding, eating only small amounts and rarely doing significant damage even to a single shoot. However, the conspicuous caterpillars of the milkweed tussock moth aggregate on a shoot and can denude it of leaves, leaving only the skeleton of the larger veins. Interestingly, tussock moth caterpillars, which sequester cardiac glycosides, metamorphose into inconspicuous (cryptic) nocturnal moths, that do not sequester appreciable amounts of cardiac glycosides (Barber & Conner, 2007).
As an adult, the monarch butterfly migrates south. The monarchs east of the Mississippi fly as far as 4,800 km to Mexico where they overwinter. “Amazingly, these butterflies fly from their summer breeding range, which spans more than 100 million ha [hectare], to winter roosts that cover less than 20 ha, often to the exact same trees, year after year” (Solensky, 2004, p. 79). The expansive extent of the summer range corresponds to the range of common milkweed and a number of other milkweed species. Along their route of migration, they feed on milkweed nectar and the nectar of other flowers. Their range contracts to the small overwintering area in Mexico, where they are temporally and spatially separated from milkweed. However, they still carry small traces of the plant in their bodies through the cardiac glycosides. The next spring they migrate back north and many of these adults mate, lay eggs, and die in the southeastern U.S. Their offspring feed on southern milkweeds, metamorphose, and the adults fly north to find common milkweed flowering in the northern summer. The life cycle begins anew.
While the life history of an individual monarch can span nearly a whole continent, the life history of a red milkweed beetle (Tetraopes tetraophthalmus) is much more tightly linked to a local common milkweed population. I will describe this relation is some detail (Agrawal, 2005; Farrell & Mitter, 1998; Hartman, 1977). About the time a colony of milkweeds begins to flower, bright red milkweed beetles crawl out of the ground and spread out onto milkweed shoots — an insect version of flowering. They crawl around on the plants and may fly short distances. They generally don’t leave the area of the colony. They begin feeding — partly on leaves (Figure 11), but mostly on flowers. When a milkweed colony is at a high point in flowering, the red milkweed beetle has its peak in population density. The adults live for about three to four weeks, which correspond to the main phase of flowering. The synchrony between adult beetle and flowering milkweed is striking. In a colony that flowers later in the year, the beetles emerge later. It could be that the temperature of the soil helps to coordinate this synchrony, since both shoot development in milkweeds and pupation in the milkweed beetle are temperature-dependent (Hartman, 1977).
The beetles mate and the female moves to a nearby grass plant or other hollow-stemmed old-field plant and nibbles a hole in the stem, crawls inside and lays her eggs. This is the one phase of the life cycle that is not dependent on milkweeds. When the eggs hatch, the larvae crawl down into the ground and move to the milkweed rhizomes. There they begin to feed, both on the inside and outside of the rhizomes. They feed exclusively on milkweed rhizomes. They can do considerable damage to short sections of a rhizome, but never have a significantly detrimental effect on a colony as a whole. While the colorless larvae are busily feeding below ground on the rhizomes, the fiery red adults have died. The larvae feed until early fall, when they move out of the rhizomes and overwinter in the soil, near the rhizomes, as large pre-pupae. They do not feed during this time. Both milkweed and pre-pupae are quiescent during the winter. Only when the soil reaches a temperature of about 17 to 18 degrees Celsius does the pre-pupa become active — not through movement or feeding, but through metamorphosis. It forms a pupa out of which the adult beetle soon emerges. It breaks through the cocoon and digs its way out of the soil to emerge in a forest of milkweeds, where it begins to feed. The next adult generation begins its short life.
When we reflect on such relationships between two kinds of organisms, a plant and an animal, the boundary between the two begins to dissolve. We can no longer think of the plant without the animal or the animal without the plant. Normally we think of the plant and the animal that feeds on it as two separate organisms that interact. It is challenging, in fact, not to describe them in such terms. But we can ask the question, “Where do organisms end?” (Holdrege, 2000). Clearly, the milkweed is unthinkable without its animal associations, just as the animals cannot be described or understood without the milkweed. Milkweed’s pollination is wholly dependent upon insects just as many insects are dependent upon milkweed for food and reproduction. Therefore, we must transcend taxonomic boundaries when we look at the fullness of an organism’s life. We can begin to see organisms as intersecting relationships that are part of the greater web of life. In the case of common milkweed, this is especially evident, since even some of its physical substances (cardiac glycosides) become a part of various animal species.
From an evolutionary perspective, we need to imagine that the lives of common milkweed and its specialist insects have been related to each other for a long period of time — going back to the mid-Tertiary in the case of the red milkweed beetle (Farrell & Mitter, 1998). They have co-evolved and have a history together — they belong to each other or are part of each other. One of the key realizations of an ecological-evolutionary perspective is that what appear today to be separate entities are in fact interconnected. As one biologist has stated, “The process of co-evolution between plants and their natural enemies — including viruses, fungi, bacteria, nematodes, insects and mammals — is believed by many biologists to have generated much of the Earth’s biological diversity” (Rausher, 2001, p. 857). That this diversity is an expression of the interconnectedness between life forms is what we begin to understand and to appreciate when we concern ourselves with the life histories of intersecting organisms.
Summarizing Picture
When you see an old field, the robust common milkweed plants stand out among the much sleeker grasses, asters, or goldenrods. Common milkweed has thick stems and expansive leaves that in shape and size look more like the leaves of a plant growing in shady woods than in a sunny old field. In the warm summer days of late June and through much of July, the large spherical heads of flowers unfold on the upper part of the stems. The individual flowers are actually quite large for a field plant and they produce large amounts of concentrated nectar. Their scent spreads out into the surroundings. When in flower, a colony of milkweeds attracts — day and night — a great variety and number of insects of all different shapes and sizes. For several weeks in summer milkweed becomes a microhabitat with a singular concentration of insect life.
The flower is highly specialized. Those parts of the flower that normally are in direct contact with the air and insects — the receptive stigma and the pollen grains — are encapsulated, the stigma within the stigmatic chamber that opens to the world only through a narrow slit, and the pollen grains in the pollinia, which themselves are hidden within the chambers. Pollination becomes an intricate process of removal and insertion that is unthinkable without the intervention of insects. Only they can bring the specialized structures into the precise spatial relation the plant needs for fertilization to occur.
While the flower outwardly displays milkweed’s strong specialization in its form, all parts of the plant except the flowers produce the specialized latex sap (confirmed by S. Malcolm). Instead the flowers produce a sweet nectar. The latex sap is encountered by animals that feed on the plant. Small insects can become caught in the sticky sap. Others can be repelled by the cardiac glycosides in the sap, while still others incorporate the toxins into their own body. The life of these often vibrantly colored insects is in multiple ways closely bound up with the milkweed.
After the flowers wilt, the fruit pods begin to expand. While relatively few fruits form out of the multitude of flowers, those that develop grow large — much larger than those of other old-field community plants. The pods swell and orient themselves upward, a contrasting gesture to the globes of flowers. Each pod is full of seeds, seeds that are large and heavy. But they have the light feather-like extensions of the white comas that allow them to be carried away on a breeze when the pods split open. It is almost as if the upward pointing pods are prefiguring what is to come — the upward lift of the coma-bearing seeds that disperse into the larger environment. As with all stages of milkweed, both pods and seeds provide nourishment to insects.
One salient feature that informs milkweed is its exuberant and robust growth. Underground it spreads year to year, forming a network of thick rhizomes out of which the aboveground shoots grow. The thick shoots bring forth large, spreading leaves. All these parts of the plant contain the milky sap, which is continually produced as the plant grows and develops. A marked transformation in substance and form occurs as the many large umbels unfold in the summer light and warmth. As the stems and leaves are rich in milk sap so are the flowers rich in sweet nectar. Both the leaves and the flowers attract countless insects; milkweed is of fundamental importance to the existence of some of these creatures. In the fall, large pods form, containing many large seeds that spread out into the environment.
Milkweed is effusive and yet it is also specialized. This specialization both attracts and repels insects. Think of the sticky, toxic sap that can also be protective, or the pollination process in which insects are attracted to the nectar, but may become injured or trapped by the flower structure. Milkweed invites life, but also holds it back. There is a fascinating tension in this plant.
The Story of an Organism
I began this essay with a quote from Aldo Leopold:
All I am saying is that there is also drama in every bush, if you can see it. When enough men know this, we need fear no indifference to the welfare of bushes, or birds, or soil, or trees. We shall then have no need of the word “conservation,” for we shall have the thing itself. (1999, p. 172)
Leopold could make this bold statement because he had experienced in a clear and deep way the “drama” in every organism — how each organism is a whole that is active within the larger living context of its environment. I have tried to portray something of the drama of the common milkweed. Such a whole organism study leads both into depth and breadth. We get nearer to the specific qualities of the plant. We begin to see its uniqueness within all the details and begin to articulate those unique qualities. Inasmuch as we are able to do just that, a story of this organism’s unique way of being emerges — no other plant is the same. Becoming aware of such a story and participating in it, cannot leave us cold — we have met a unique quality in the world and the world would be poorer without it. Holistic knowing creates the basis of a moral relation to the world, for when we have experienced nature in this way, “we need fear no indifference to the welfare of bushes, or birds, or soil, or trees.”
The story of an organism always leads beyond itself to a larger web of relations with other organisms and elements of the environment. There is no isolation in the living world. We attend closely to the specific qualities, for instance, of milkweed, monarch butterfly, or milkweed beetle, and at the same time become vividly aware of how these qualities intersect and are mutually dependent. We begin to gain insight into the truly ecological nature of life.
This kind of knowing is anything but abstract, and it is not the kind of fear-arousing knowledge that informs so much of the environmental debate today. Aldo Leopold captures the essence of the problem and the task when he writes, “I have no hope for conservation born of fear. The 4-H boy who becomes curious about why red pines need more acid than white [pines] is closer to conservation than he who writes a prize essay on the dangers of timber famine” (1999, p. 165). It is engaging in concrete phenomena that gives us a relation to them that goes beyond mere information. As Leopold puts it, “we can only be ethical in relation to something we can see, feel, understand, love, or otherwise have faith in” (1989, p. 214).
References
Agrawal, A. A. (2005). Natural selection on common milkweed (Asclepias syriaca) by a community of specialized insect herbivores. Evolutionary Ecology Research, 7, 651-667.
Barber, J. R., & Conner, W. E. Acoustic mimicry in a predator-prey interaction. PNAS, 104, 9331–9334.
Baum, H. (948). Der Fruchtansatz von Asclepias syriaca. Plant Systematics and Evolution, 94: 402–403.
Berenbaum, M. (1993). Sequestered plant toxins and insect palatibility. The Food Insects Newsletter, 6(3), 1-10.
Bookman, S. S. (1981). The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification of terminology for the genus. American Journal of Botany, 68, 675–679.
Cohen, J. A., & Brower, L. P. (1983). Cardenolide sequestration by the dogbane tiger moth (Cycnia tenera; Arctiidae). Journal of Chemical Ecology, 9,521–532.
Dailey, P. J. Graves, R. C., & Herring, J. L. (1978). Survey of hemiptera collected on common milkweed, Asclepias syriaca, at one site in Ohio. Entomological News, 89, 157–162.
Dailey, P. J. Graves, R. C., & Kingsolver, J. M. (1978). Survey of coleoptera collected on the common milkweed, Asclepias syriaca, at one site in Ohio. The Coleopterists Bulletin, 32, 223–229.
Duffey, S. S., & Scudder, G. G. E. (1972). Cardiac glycosides in North American Asclepiadaceae, a basis for unpalatability in brightly coloured hemiptera and coleoptera. Journal of Insect Physiology, 18, 63–78.
Duffey, S. S., Blum, M. S., Isman, M. B., & Scudder, G. G. E. (1978). Cardiac glycosides: a physical system for their sequestration by the milkweed bug. Journal of Insect Physiology, 24, 639–645.
Dussourd, D. E. (1999). Behavioral sabotage or plant defense: do vein cuts and trenches reduce insect exposure to exudate? Journal of Insect Behavior, 12, 501–515.
Farrell, B. D., & Mitter, C. (1998). The timing of insect/plant diversification: might Tetraopes (Coleoptera: Cerambycidae) and Ascelpias (Asclepiadaceae) have co-evolved? Biological Journal of the Linnean Society 63, 553–577.
Fordyce, J. A., & Malcolm, S. B. (2000). Specialist weevil, Rhyssomatus liineaticollis, does not spatially avoid cardenolide defenses of common milkweed by ovipositing into pith tissue. Journal of Chemical Ecology, 26, 2857–2874.
Fox, C. W., & Caldwell, R. L. (1994). Host-associated fitness trade-offs do not limit the evolution of diet breadth in the small milkweed bug Lygaeus kalmii (Hmiptera: Lygaeidae). Oecologia, 97, 382–389.
Fritz, R. S., & Morse, D. H. (1981). Nectar parasitism of Asclepias syriaca by ants: effect on nectar levels, pollinea insertion, pollinaria removal and pod production. Oecologia, 50, 316–319.
Goethe, J. W. (1995). Scientfic studies. Princeton: Princeton University Press.
Hartman, F. A. (1977). The ecology and coevolution of common milkweed (Asclepias syriaca, Asclepiadacieae) and milkweed beetles (Tetraopes tetraophthalmus, Cerambycidae). Ph.D. Thesis, University of Michigan, Ann Arbor.
Helmus, M. R., & Dussourd, D. E. (2005). Glues or poisons: which triggers vein cutting by monarch catepillars? Chemoecology, 15, 45–49.
Holdrege, C. (2000). Where do organisms end? In Context, 3, 14–16.
Jennersten, O., & Morse, D. H. (1991). The quality of pollination by diurnal and nocturnal insects visiting common milkweed, Asclepias syriaca. American Midland Naturalist, 125, 18–28.
Leopold, A. (1989). A Sand County Almanac. New York: Oxford University Press. This book was originally published in 1949.
Leopold, A. (1999). For the Health of the Land. Washington, D. C.: Island Press. The quotes are from the essay “The Farmer as Conservationist,” which was originally published in 1939.
Kephart, S. R. (1987). Phenological variation in flowering and fruiting of Asclepias. American Midland Naturalist, 118, 64–76.
Malcolm, S. B. (1994/1995). Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology, 5/6, 3/4, 101–117.
Mooney, K. A., Jones, P., & Agrawal, A. A. (2008). Coexisting congeners: demography, competition, and interactions with cardenolides for two milkweed-feeding aphids. Oikos, 117, 450–458.
Morse, D. H. (1982). The turnover of milkweed pollinia on bumble bees, and implications for outcrossing. Oecologia, 53, 187–196.
Pleasants, J. M. (1991). Evidence for short-distance dispersal of pollinia in Asclepias syriaca L. Functional Ecology, 5, 75–82.
Price, P. W.,& Wilson, M. F. (1979). Abundance of herbivores on six milkweed species in Illinois. American Midland Naturalist, 101, 76–86.
Prysby, M. D. (2004). In Oberhauser, K. S., & Solensky, M. J. (Eds.), The Monarch Butterfly: Biology and Conservation (pp. 79–83). Ithaca: Cornell University Press.
Sage, T. L., & Williams, E. G. 1995). Structure, ultrastructure, and histochemistry of the pollen tube pathway in the milkweed Asclepias syriaca L. Sex Plant Reprod, 8, 257–265.
Solensky, M. J. (2004). Overview of monarch migration. In Oberhauser, K. S., & Solensky, M. J. (Eds.), The Monarch Butterfly: Biology and Conservation (pp. 79–83). Ithaca: Cornell University Press.
Southwick, E. E. (1983). Nectar biology and nectar feeders of common milkweed, Asclepias syriaca L. Bulletin of the Torrey Botanical Club, 110, 324–334.
Wilbur, H. M. (1976). Life history evolution in seven milkweeds of the genus Asclepias. The Journal of Ecology, 64, 223–240.
Willson, M. F., & Rathcke, B. J. (1974). Adaptive design of the floral display in Asclepias syriaca L. American Midland Naturalist, 92, 47–57.
Woodson, R. E. (1954). The North American species of Asclepias L. Annals of the Missouri Botanical Garden, 41, 1–211.
Wyatt, R. & Broyles, S. B. (1994). Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics, 25, 423–441.
Wyatt, R., Browles, S. B. & Derda, G. S. (1992). Environmental influences on nectar production in milkweeds (Asclepias syriaca and A. exaltata). American Journal of Botany, 79, 636–642.
Copyright 2010 by The Nature Institute. All photographs and drawings are by Craig Holdrege.
Excerpts from an earlier version of the essay were published in In Context (#22, Fall 2009, pp. 7-11; #23 Spring 2010, pp. 3-6; #24, Fall 2010, pp. 3-7).